The long-range goal of our research is to enhance the lives of individuals
with intestinal dysfunction. Efforts focus on understanding
the regulation of small intestinal function by various nutrients and
intestinal-specific peptides, and our laboratory’s publications
demonstrate versatility in testing hypotheses addressing fundamental
issues in gastrointestinal physiology. In addition to our studies
conducted in both human subjects and cell culture, our hypotheses surrounding
the regulation of gastrointestinal function have led us to establish
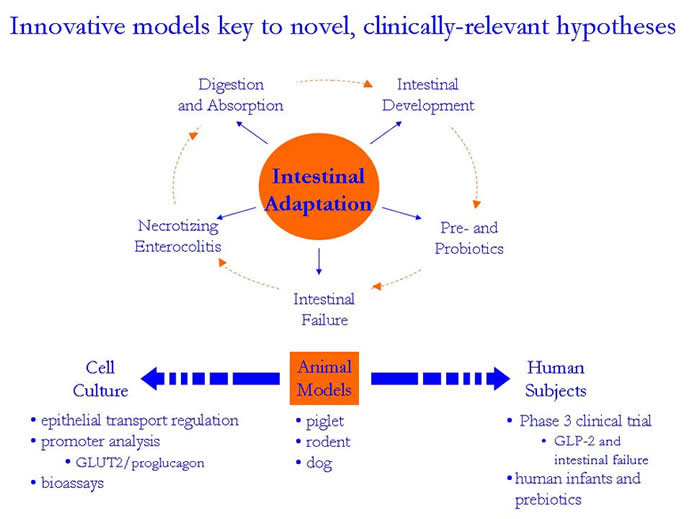
innovative animal models simulating a spectrum of clinical scenarios
involving intestinal adaptation (Figure 1). A brief discussion
of the impact of our research is described
on the following pages.
Premature infants are at risk for short bowel
syndrome secondary to necrotizing enterocolitis (NEC),
whereas adults who are traumatically injured face intestinal resection
following non-occlusion small bowel necrosis (NOSBN). Aggressive
enteral nutrition and poor intestinal perfusion are hypothesized to play an important
pathogenic role in both NEC and NOSBN. Our research has tested
the novel hypothesis that during intestinal hypoperfusion, or reduced blood flow,
enteral nutrients may increase oxygen demand beyond that available, potentially
increasing intestinal hypoxia and impairing small intestinal function (Tappenden,
2003). Our results indicate that hypoperfusion alters the
transport of specific nutrients across the intestine and this has important
implications for specialized nutrition support provided by
neonatologists, intensivists and nutritionists. Using newly developed
models of NEC in the clinically-relevant neonatal piglet, the cellular mechanisms
underlying the differential regulation of nutrient transport during hypoperfusion
are being examined. This information is critical for assuring that the
composition of enteral nutrients provided to the hypoperfused intestine is optimized
to prevent further impairment in GI function.
When NEC develops, massive small bowel resection is typically performed
to remove the necrotic intestine. However, this intervention often
results in short bowel syndrome (SBS), rendering the infant with inadequate intestinal
surface area for digestion and absorption of
orally consumed nutrients. My laboratory has developed a neonatal piglet
model that combines an 80% massive small bowel resection with intravenous
nutrition, or total parenteral nutrition TPN (Tappenden et al., 2003),
thereby establishing an excellent pre-clinical model for investigating
therapeutic modalities for SBS (Bartholome et al., 2004; Albin et al.,
2003a; 2003b). This surgical model has become the focus for a NIH-funded
research project aimed at understanding the underlying mechanism(s)
whereby short-chain fatty acids (SCFA) modulate intestinal adaptation
in neonates receiving TPN. Work from our laboratory, has
determined that the supplementation of parenteral nutrition with SCFA
enhances structural and functional adaptation in neonatal piglets (Albin
et al., 2003a; 2003b; Bartholome et al., 2004) following massive small
bowel resection. It appears that butyrate is the SCFA responsible
for augmenting structural aspects of intestinal adaptations by increasing
proliferation and decreasing apoptosis as early as 4 hours post-resection
(Bartholome et al., 2004). Current efforts focus on whether butyrate
mediates these effects directly or involves a mechanism relating to
induced expression of the intestinotrophic peptide, glucagon-like peptide-2
(GLP-2; Bartholome et al., 2005; Mangian et al., 2006). The rationale
that underlies this research is that if the
role and underlying mechanism(s) whereby short-chain
fatty acids modulate intestinal adaptation in neonates receiving TPN is
understood, nutritional formulas could be optimized to promote intestinal
adaptation in children with short-bowel syndrome and reduce their long-term
dependence on TPN.Regardless of the experimental model/species studied,
we have noted that a very consistent response to SCFA administration is
upregulation of the brush-border glucose transporter, SGLT-1, and basolateral
hexose transporter, GLUT2. However,
our research indicates that SCFAs appear to utilize
different cellular and molecular mechanisms to induce the increase in
SGLT-1 or GLUT2 activity (i.e., an increase in mRNA abundance of SGLT-1
is not observed as it is for GLUT2). To study these acute observations and underlying
mechanism(s), we have developed both in situ and ex vivo experimental
models with neonatal piglet intestine. We have recently
made thenovel observation that ileal tissue exposed to butyrate
for as little as 15 minutes results in a 6-fold increase in glucose
transport via SGLT-1 (Chung and Tappenden, 2005). Our
laboratory is currently investigating whether the
mechanism of the butyrate-mediated response is via facilitating recruitment
of intracellular pools of SGLT-1 to the brush-border membrane. To examine the regulatory mechanism
whereby SCFAs increase GLUT2 mRNA abundance, reporter assays are
conducted with differentiated Caco-2BBe monolayers transfected with
the GLUT2 promoter and indicate that transcription of the GLUT2 promoter
is initiated by butyrate (Mangian et al., 2006). Understanding
these mechanisms provides valuable insight into treatment
modalities and markers of therapeutic efficiency for use in individuals
with intestinal failure. This
work also demonstrates our efforts to support the hypotheses being
tested in novel in vivo models with focused, mechanistic in vitro experiments
thereby increasing the breadth of experimental data generated.
In addition to our pre-clinical animal models and in vitro studies, we
are conducting studies with human subjects with SBS to investigate the therapeutic
effectiveness of a new gastrointestinal peptide analog, teduglutide (Jeppesen
et al., 2005; Tappenden et al., under review). These efforts are
currently the focus of a Food and Drug Administration Phase III clinical trial
wherein samples from clinical investigators around the world send mucosal biopsies
from TPN-dependent patients with intestinal failure to my laboratory for assessment
of the structural and functional adaptations induced by teduglutide therapy. The
leadership of our research group in the area of intestinal failure has been recognized
by the National Institutes of Health (NIH) wherein they invited Dr. Tappenden
to chair a 2-day research workshop in 2004 titled ‘Intestinal Failure:
Current and Emerging Therapies Including Transplantation’ (Figure 2). This
invitation allowing Dr. Tappenden to assemble a faculty of 24 international experts
and drew approximately 300 clinical and basic researchers from 9 countries. The
culmination of these efforts allowed Dr. Tappenden to serve as Associate Editor
for Gastroenterology, the leading journal in this field, wherein the proceedings
of this symposium were recently published (2006, volume 130). In
conclusion, our
numerous efforts in both basic and clinical research, in addition to NIH-sponsored
workshops and publications, promise to realize our long-term goal of enhancing
the lives of patients with intestinal dysfunction.
Consistent
with our overall research focus, we have developed models for both parenteral
and enteral (gastrostomy and jejunostomy) nutrient administration, as well
as diarrheal diseases (salmonella infection) in neonatal piglets (Correa-Matos
et al., 2003; Milo et at., 2004). Various collaborators
have sought this expertise to examine nutrient formulas
with a range of characteristics on the function and structure of the gastrointestinal
tract. Findings
to date indicate that both parenteral and enteral solutions can be formulated
to enhance intestinal function. Furthermore, because of our commitment
to understanding the cellular alterations induced
by the optimal administration of specialized nutrition
support, our laboratory is currently identifying molecular and
functional markers that can be used to assess the efficacy of therapeutic
strategies for individuals with intestinal dysfunction.
In summary, by understanding the cellular adaptations, molecular mechanisms and
applicability to human populations, work from our research group is impacting
the medical nutrition therapy provided to patients with intestinal failure. Ultimately,
we expect to significantly enhance their quality of life by reducing their dependence
on long-term TPN.
|